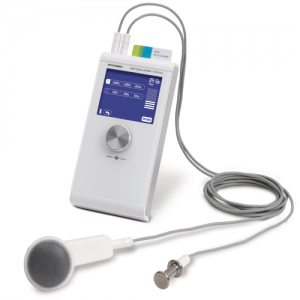
Physiomed Magcell Arthro
Pain-alleviating and movement-promoting effect for osteoarthritis
Product description
MAGCELL® ARTHRO significantly improves general symptoms (WOMAC total score) and individual scores for pain, stiffness and daily activity in osteoarthritis (ARC criteria II and III). The therapy can be applied several times daily as a complementary treatment without side effects and may thus help to reduce intake of pain medication.
Features
- Very easy one-button operation
- Battery-driven
- Optical and acoustic function control
- Automatic switch-off at the end of the therapy period
- Electrode-free electrotherapy for therapists and patients
- Pulsating electromagnetic fields (PEMF)
- Field strength more than 1000 gauss
- Effective treatment concept due to repeatable short-treatment periods
- Through-textile treatment (even through shoes)
WOMAC total score
In a randomised controlled study on the effect of MAGCELL® ARTHRO for knee arthritis with osteoarthritis level 2.8±0.8 (American College of Rheumatology criteria) at the primary clinical end point (WOMAC total score) median increase of 0.7 P (non-significant) was recorded in the placebo group between T0 and T1 (18 days), yet in the MAGCELL®-group a significant local decrease of 21.8 P. During the study no undesirable incidents or side effects occurred related to therapy.
Therapy information
Through a patent-registered, innovative procedure, MAGCELL® ARTHRO is able to create exceptionally strong pulsating magnetic fields of up to 100 mT (1000 gauss) with selective frequency ranges despite its small size. Time-variable magnetic fields can function as transfer media for electric fields. Providing that – like MAGCELL® ARTHRO – the field force is sufficiently high, electro-magnetic fields can be induced in the tissue beyond the internationally recognised threshold for biological effectiveness. Through the possibility of direct application and the resulting close distance of the magnetic field to the application area, MAGCELL® ARTHRO also ensures minimum loss of magnetic field strength, transporting it deep into the tissue layers (electrode-free electrotherapy). Its battery operation and thus constant readiness enables the execution of a therapy programme through repeated short treatments in contrast to treatments previously only offered in doctor’s surgeries.
MAGCELL® ARTHRO is ideal for painful hip, knee and jaw joint arthritis, Hallux rigidus and valgus and a range of other arthritic conditions. Clinical studies on the complementary use of MAGCELL® ARTHRO for acute knee arthritis (1,2) have shown that amongst others, the following clinical effects can be achieved:
- Significant relief from inflammation pain
- Improvement in joint movement
- Reduction of knee joint circumference
- Significant reduction of WOMAC index
Magnetic field therapy is available in a range of types and appearances. Various forms of therapy do not have sufficient scientific proof of their effectiveness. The MAGCELL® ARTHRO medical product cannot be compared with ‘pulsating signal therapy (PST)’, ‘MultiBioSignal Therapy (MBST)’, ‘Nuclear Magnetic Resonance Therapy’, ‘TENS' or other transmission forms such as magnetic field mats or coils.
MAGCELL® ARTHRO works with therapy parameters investigated in empirical and clinical research. There are no known side effects or intolerance associated with the use of MAGCELL® ARTHRO. Based on a large amount of patient feedback, regular therapy may enable reductions in the dosage of medication, such as non-steroidal anti-rheumatic agents.
------------------------
1 Hitrov N, Portnov V (2008): MAGCELL® ARTHRO in der Behandlung von Arthrose im Kniegelenk. Die Naturheilkunde 3:25-27.
2 Wuschech H., von Hehn U., Mikus E., Funk R.H. (2015): Effects of PEMF on patients with osteoarthritis: Results of a prospective, placebo-controlled, double-blind study. Bioelectromagnetics 36(8), 576–585.
| Specifications | |
| Current Consumption | 0.35 A |
| Electrical Protection Class | 2, type BF |
| Input Current | 1.3 ADC |
| Input Voltage | 7.2 VDC (4 Cells) |
| Load Impedance | 10 M Ohm |
| Main Power Frequency | 50/60 Hz |
| Maximum Output Voltage | 400 V |
| Output Frequency | 5-250 Hz |
| Power Connection | 100 - 240 VAC +-10% |
| Power Supply | 4 x NiMH- Battery Size AA, 1.2 V |
| Product Dimensions (cm) | 10 x 3.1 x 19 |
| Product Weight (kg) | 0.7 |
| Shipping Details | |
| Availabillity | In Stock |
| Delivery Time | 9 - 15 Days |
| Shipping Dimension (cm) | 20 x 13.1 x 29 |
| Shipping Weight (kg) | 2 |